The FDA Just Fast Track Approved a New Tumor Infiltrating Lymphocyte Treatment (TIL) Called Amtagvi for Advanced Melanoma
It's the first approval of its kind for skin cancer, potentially opening the floodgates for a new generation of treatments but at what cost?
The FDA's use of the accelerated approval pathway has shown significant trends and shifts over the years. Initially, the pathway was primarily used to expedite approvals for drugs treating severe conditions like HIV and cancer, remember AZT? That drug ended up being so toxic that it killed untold amounts of people afflicted with HIV that may not have otherwise perished from the disease. Look up what happened to Arthur Ashe if you don’t believe me.
From 1992 to 2010, a substantial proportion of accelerated approvals were for HIV (39.7%) and cancer (35.6%) treatments. However, from 2010 to 2020, there was a marked shift towards oncology, with 85% of accelerated approvals targeting cancer treatments.
A significant increase in the use of expedited approval programs, including accelerated approval, was noted between 2000 and 2018. This trend is evident from the rising annual number of new drug approvals, which spiked notably from an average of 23 new molecular entities (NMEs) per year from 2000 to 2010 to 35 in 2011, reaching up to 59 by 2018
Moreover, the approval rate for first-in-class drugs, which often use the accelerated pathway, has also increased. For example, in 2021, a notable number of first-in-class drugs received accelerated approval.
According to an FDA Report:
“14 of 50 drugs (28%) using the pathway. In contrast, 12 of 53 drugs (22.6%) in 2020, 9 of 48 drugs (18.7%) in 2019, and 4 of 59 drugs (6.7%) in 2018 received the Accelerated Approval designation.”
These trends indicate a significant reliance on the accelerated approval pathway to bring drugs to the market more swiftly. These approvals are couched as potentially life saving to patients who have no other options, tugging at a docile public’s heart strings.
However, closer scrutiny of the approval process reveals that there is much more to this story than just “saving lives”. Many of these “fast tracked” drugs have shown themselves to be very problematic to those tasked with taking them. Often leaving their health in much worse shape than before and sometimes killings people, as you will soon see.
Made by Iovance Biotherapeutics, the new treatment we will be examining, called Amtagvi, will cost already cash strapped patients no less than $515,000 per treatment. The approval by the FDA was based on the data from a Phase 2 trial while a confirmatory Phase 3 trial is still ongoing. As of right now, it can only be used by people who have melanoma that cannot be removed surgically or has spread and has been treated with other drugs. So we are talking about people with already highly depleted immune systems due to the previous treatment.
If you search online, you will find legacy media fawning over the approval touting its 31.5% responder rate, meaning the tumor had a reduction in size in 31.5% of recipients. We will get to this later.
The main thrust of this approval and the reason it is being celebrated is that it opens potential floodgates for a new generation of Tumor Infiltrating Lymphocyte (TIL) therapies. The Washington Post had this to say:
“Though the condition is uncommon, cancer experts say winning FDA approval could mark the beginning of a potent new weapon against far more common tumors.”
However, as much as investors and the biopharmaceutical industry love the 47% Iovance stock increase since the announcement, the celebration may be premature because the FDA also issued a black box warning for the treatment.
Given this warning and since this is being billed as ushering in a new era in cancer treatments, I decided it would be prudent to have a look at the studies this approval is based on by reviewing the package insert provided by the FDA. Just to see how “safe & effective” this new revolutionary TIL treatment truly is.
A 31.5% respondent rate doesn’t seem all that revolutionary to me, especially if you are charging a whopping $515,000 for a single treatment. If I am paying that much, the responder rate needs to be much higher than that, especially considering it has a black box warning which we are going to review.
Once we have examined the trial data we will then break down the Conflicts of Interests submitted by the authors, which I think will bring my suspicions into focus about this drug’s approval being used to usher in a cottage industry of these new extremely profitable TIL treatments.
The sociological forces surrounding drug approvals are far too often ignored as if human nature stops being human nature because it is drug research. The propensity for bias that aligns with the potential social and financial capital to be gained has to be taken into consideration. Especially in light of the fraud and deception both the pharmaceutical industry and the government have perpetrated in recent decades.
Let’s be honest, here we have a highly toxic novel treatment being given to individuals who haven’t responded well to other treatments. It costs these people $515K on top of their other medical bills and has a mortality rate of 7.5%.
This is why closer scrutiny of COI statements is needed today. It’s as if just because they tell you they have conflicts of interest that is supposed to magically absolve them for their conflicts of interest. This is the logic of a vampire. Speaking of which let’s examine the level of “fair warning” the vampires deem acceptable.
THE BLACK BOX WARNING
Please note, that this and all subsequent screenshots are taken directly from the package insert of Amtagvi unless otherwise noted. As you can see the warning given is quite serious, all of these conditions are not to be trifled with and I am going to go through each one so that you can gain an understanding of what they entail and the potential implications to the health of anyone who receives this treatment. We will then continue to the Clinical Trial Data and parse what it actually means instead of just relying on what the FDA and Iovance’s press release says like the rest of the world.
A "black box warning" is the most serious warning that the U.S. Food and Drug Administration (FDA) can require for prescription drug labeling. It indicates that medical studies have demonstrated that the drug carries a significant risk of serious or even life-threatening adverse effects. The warning is presented in a prominent black box to ensure that both prescribers and patients are aware of the risks.
Here are the conditions they are warning the public about.
“Severe cytopenia, internal hemorrhage, severe infections, and be sure to monitor for cardiopulmonary and renal functions.”
Interestingly, I found an article on cancer.gov about the FDA approval, and nowhere in it did it mention the black box warning. Here is how it characterized side effects.

“All participants in the trial had side effects caused by the treatment, Dr. Wermke reported. Most, however, were not dangerous and were largely caused by the chemotherapy given before the lifileucel infusion and IL-2 given afterward. The most common included anemia, high fevers, and substantial drops in levels of platelets and certain white blood cells.”
This is a patently false characterization of the side effects laid out in the study themselves as well as the package insert. These willful misrepresentations could be due to the fact that the National Cancer Institute entered into a “cooperative research agreement” with Iovance way back in 2011.
“In 2011, NCI entered into a cooperative research agreement with Iovance to further develop this particular TIL therapy, including conducting larger clinical trials and developing a manufacturing infrastructure, paving the way for FDA approval.”
“It’s been a long time coming, Dr. Rosenberg acknowledged. The new approval “is a big step,” he said, one which demonstrates that “cellular therapy is joining the mainstream of cancer treatment.”
In fact, the NCI’s stake in TIL goes back further than 2011, according to a news release by the H. LEE MOFFITT CANCER CENTER & RESEARCH INSTITUTE:
“Tumor-infiltrating lymphocyte therapy has been around for decades. Pre-clinical studies evaluating its efficacy began in the early 1980s at the National Cancer Institute (NCI). James J. Mulé, IPh.D., a world-renowned immunologist and associate center director of Translational Science at Moffitt, brought TIL research to the cancer center in 2003.”
Over 40 years in the making.
Cooperative Research Agreements (CRA) are used widely between the government and private companies. It usually means parties will contribute resources, expertise, and possibly personnel to conduct specific research. For example, a government laboratory might offer scientific expertise and specialized equipment, while the pharmaceutical company could provide additional scientific talent and funding.
The agreement outlines specific research goals, which are often aimed at developing new drugs, improving existing treatments, or enhancing manufacturing processes. These goals are clearly defined to ensure that both parties are aligned in their expectations and contributions.
CRAs usually specify how IP rights are handled. Typically, the pharmaceutical company may be granted the first option to negotiate licenses for any inventions that result from the collaboration. Both parties usually protect their pre-existing IP while sharing rights to new discoveries made during the partnership.
The agreements include confidentiality clauses that prevent the sharing of sensitive information beyond the scope of the partnership. There are often provisions related to the publication of research findings, where the government may retain the right to publish results after a certain period. The agreement might also include stipulations on how data is shared between the parties during and after the project.
The NCI’s decidedly muted tone on the black box warning and misrepresentation of overall side effects are no doubt due to the investment it has placed into this research for the past 40 years. This is illustrative of just how intertwined our “public-private partnerships” (P3) have truly become.
Here is a local news segment touting it as the next big thing in cancer treatment.
“We think this is only the start.”
The “Revolutionary Treatment” Regime
This is how this treatment works in lay terms. They are basically taking a piece of a person's tumor and then isolating the Tumor Infiltrating Lymphocytes (TIL), reproducing them in large numbers, and then putting them back into the patient via an infusion.
Tumor-infiltrating lymphocytes (TILs) are defined as lymphocytes that directly oppose and/or surround tumor cells. Here is a depiction that Iovance Biotherapeutics provides to describe the process.
This is a bit more detailed depiction of how TIL therapy is conducted to give you a visual sense of the process.
Now that we understand the basic idea behind this therapy let’s have a look at the details that precipitated the black box warning.
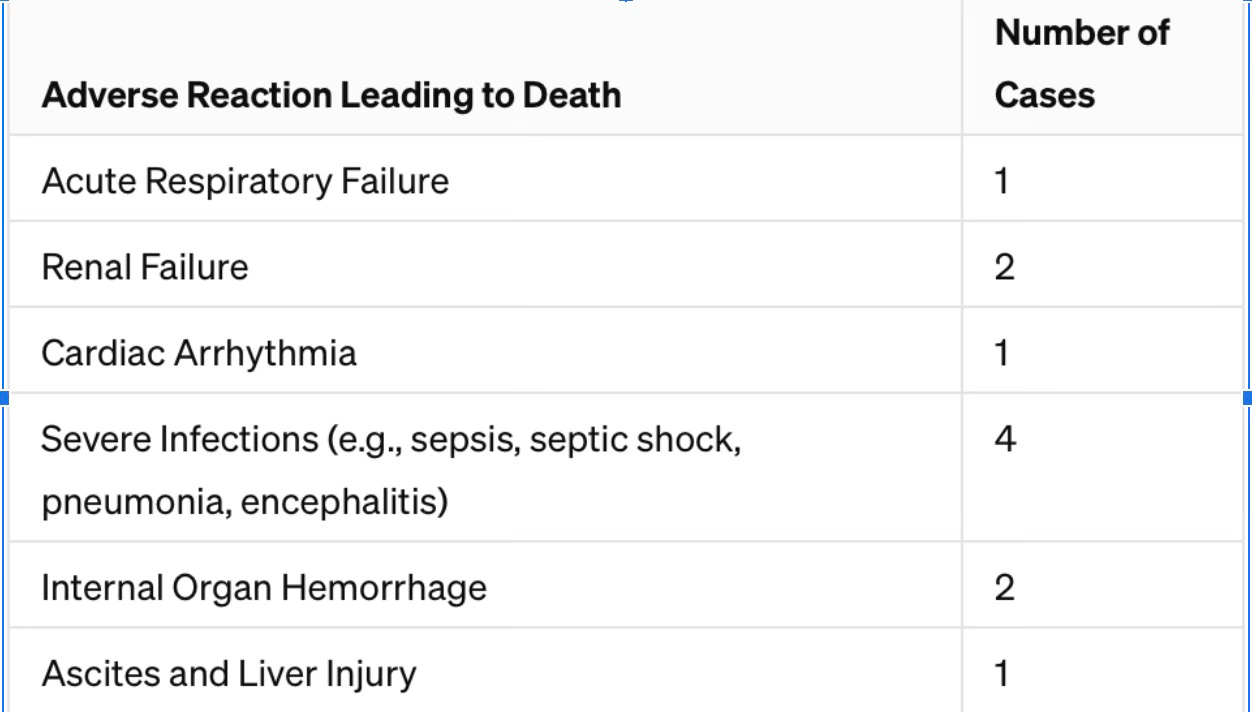
Under the section labeled “Treatment-Related Mortality”, it tells us that out of 160 people who received AMTAGVI, 7.5% of them died because of issues related to the treatment. This means out of every 100 people treated with AMTAGVI, about 7 or 8 people died due to complications from the treatment.
These complications included serious infections like sepsis (a severe reaction to an infection), pneumonia (lung infection), and encephalitis (brain inflammation); bleeding inside the body, such as in the abdomen or brain; kidney failure; breathing problems; heart rhythm problems; excessive fluid in the abdominal area; liver damage; and failure of the bone marrow to produce blood cells.
If you recall the article published by the National Cancer Institute blamed all of the side effects on the chemotherapy required prior to the infusion. This is an extremely convenient conclusion to make, isn’t it? Especially when we consider the financial interest the NCI has with its partner Iovance.
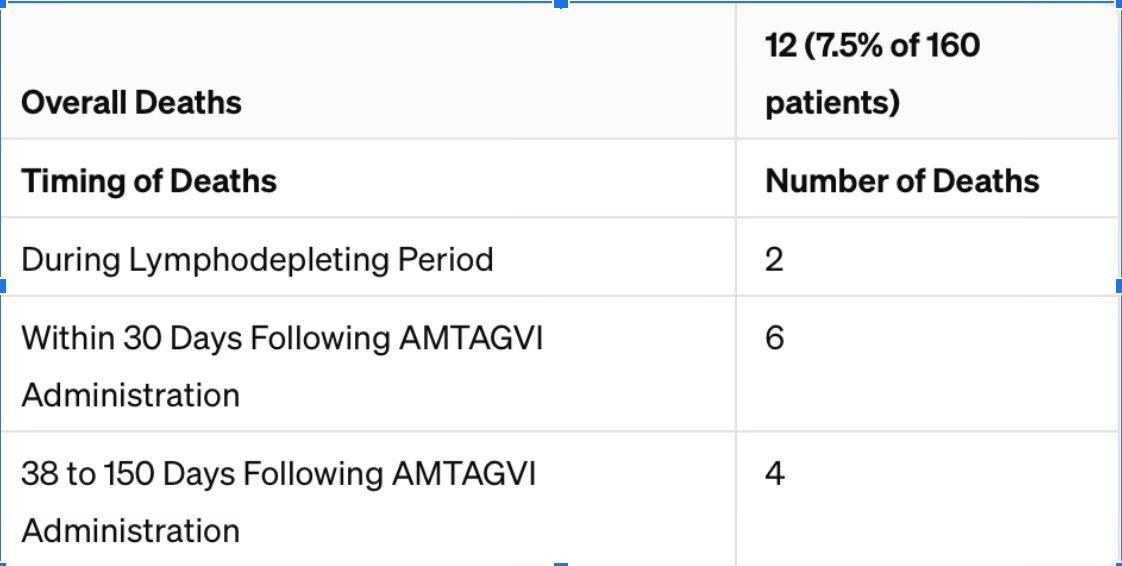
Let me get this straight, the drug killed 7.5% of the patients? 12 out of 160 people died directly because of taking it and the FDA thinks that is promising? Here is a breakdown of these deaths.
When Deaths Occurred:
2 patients died during the initial phase where the treatment weakens the immune system (this is called lymphodepletion).
6 patients died within the first 30 days after receiving AMTAGVI.
4 patients died between 38 to 150 days after getting the treatment.
Causes of Death: The deaths were due to severe side effects, including:
Serious infections like sepsis (a life-threatening response to infection), pneumonia (lung infection), and encephalitis (brain inflammation).
Bleeding inside the body, including in the abdomen and brain.
Kidney failure, where the kidneys suddenly stop working.
Respiratory failure, which means the lungs couldn't provide enough oxygen to the body or remove carbon dioxide.
Heart rhythm problems (arrhythmia), which can be life-threatening.
Accumulation of fluid in the abdomen (ascites), which can be due to various serious conditions.
Liver damage, which can affect the liver's ability to function.
Bone marrow failure, meaning the bone marrow doesn't produce enough new blood cells.
Severe Side Effects Abound with Amtagvi
SEVERE CYTOPENIA
According to what's been observed, almost half of the melanoma patients (45.5%) treated with AMTAGVI had prolonged severe cytopenia, meaning a severe drop in their blood cells that didn't get better to a milder condition or lasted for more than 30 days after the treatment.
Specifically, the types of blood cell count drops seen were:
Thrombocytopenia: A decrease in platelets, which help with blood clotting (30.1% of patients).
Lymphopenia: A decrease in lymphocytes, a type of white blood cell important for immune response (19.9% of patients).
Neutropenia: A decrease in neutrophils, white blood cells that fight infection (17.3% of patients).
Leukopenia: A general decrease in white blood cells (14.7% of patients).
Pancytopenia: A decrease in all types of blood cells (1.3% of patients).
When people are treated with AMTAGVI, there's a risk that they might experience bleeding inside their body aka Internal Hemorrhaging, specifically within the abdomen (the area that includes the stomach and other organs) or the brain. This kind of internal bleeding is very serious and can be life-threatening. In fact, such bleeding has been linked to the deaths of at least two people who were treated with AMTAGVI.
One reason this bleeding can happen is because of a condition called thrombocytopenia, where the patient has a lower number of platelets in their blood. Platelets are tiny cells that help blood to clot, so if there aren't enough of them, bleeding can happen more easily.
SEVERE INFECTION
Severe infections were also associated with the treatment. Some of these infections were so severe they could lead to death. About 26.9% of melanoma patients treated with AMTAGVI had some level of infection because of the treatment. When we talk about "Grade 3 or higher infections," it means infections that are quite severe. Out of these, 13.5% of patients had serious infections, including 10.9% where the cause of the infection wasn't identified and 3.8% where the cause was known.
Febrile neutropenia is considered an emergency in medical care, especially in patients with cancer or those receiving chemotherapy, because their immune systems are already weakened, making them more susceptible to infections. Treatment typically involves hospitalization, administration of broad-spectrum antibiotics to fight potential infections, and other supportive care measures to help the patient recover while the underlying cause of the neutropenia is addressed.
Heart Related Issues
In a group of 156 patients who received AMTAGVI, about 9% (which is 14 people) had serious heart-related issues because of the treatment. These problems included:
Tachycardia: A faster than normal heart rate.
Atrial fibrillation: An irregular and often rapid heart rate that can increase the risk of strokes, heart failure, and other heart-related complications.
Arrhythmia: Any irregular heartbeat.
Acute myocardial infarction: Also known as a heart attack, where blood flow to the heart is blocked.
Cardiac ventricular thrombosis: A blood clot in the heart's ventricles, which are the two lower chambers of the heart.
Cardiomyopathy: A disease of the heart muscle that makes it harder for the heart to pump blood to the rest of the body.
QT-prolongation: A condition that can cause unpredictable changes in the heart rhythm, which might lead to sudden, dangerous heartbeats.
One person who received AMTAGVI died due to cardia arrhythmia.
Clinical Trials Experience
156 adults who have unresectable or metastatic melanoma were given AMTAGVI. This treatment wasn't given alone; it was part of a combination that also included three other treatments: cyclophosphamide, fludarabine, and IL-2 (also known as aldesleukin).
Unresectable means the cancer couldn't be removed with surgery.
Metastatic means the cancer has spread from where it originally started to other parts of the body.
Cyclophosphamide
Cyclophosphamide is a type of chemotherapy medication. It works by slowing or stopping the growth of cancer cells in the body. Cyclophosphamide is used to treat various types of cancer by damaging the DNA of cancer cells, which makes it hard for them to divide and grow.
Some adverse reactions associated with it are bone marrow suppression, nausea/vomiting, hair loss, bladder irritation and it can effect fertility.
Fludarabine
Fludarabine is another chemotherapy drug, but it's often used to treat certain types of blood cancers, such as chronic lymphocytic leukemia (CLL) and some types of non-Hodgkin's lymphoma. It works in a similar way to cyclophosphamide, by interfering with the DNA inside cancer cells, which prevents them from multiplying.
Some adverse reactions include immunosuppression, neurological effects, bone marrow suppression and nausea/fatigue.
IL-2 (Aldesleukin)
IL-2, or aldesleukin, is different from the first two treatments because it's a type of immunotherapy rather than chemotherapy. IL-2 stands for Interleukin-2, which is a naturally occurring protein in the body that plays a role in activating the immune system. As a treatment, aldesleukin is a synthetic form of this protein and works by boosting the body's own immune response to help fight cancer.
Some adverse reactions include capillary leak syndrome, high fever and chills, heart problems and depression.
In this study:
The average age of the people involved was 56, but they ranged from 20 to 79 years old.
About 54% of the participants were men.
Before the study started, the health status of the patients was measured using something called the ECOG performance status, which helps to describe a patient's level of functioning.
68.6% of the patients were graded as ECOG 0, meaning they were fully active, able to carry on all pre-disease performance without restriction.
31.4% were graded as ECOG 1, meaning they were restricted in physically strenuous activity but could walk and carry out light or sedentary work.
EFFICACY ENDPOINTS
It is important to understand how Efficacy was established for this study, in other words what is considered a success? Let’s break down these terms to get a better understanding of what they are looking for.
It is based on something called Objective Response Rate (ORR), Duration of Response (DoR) and Median Time to Initial Response.
The Objective Response Rate (ORR) is a way to measure how well a cancer treatment works. Specifically, it looks at the percentage of patients whose cancer shrinks or disappears after treatment. If a treatment has a high ORR, it means that a significant number of patients had their cancer reduce in size, which is a good sign that the treatment is effective.
The Duration of Response (DoR) is about how long the positive effects of the treatment last. After a patient's cancer responds to treatment by shrinking or disappearing, the DoR measures how long these benefits continue before the cancer starts growing again or becomes noticeable. A longer DoR means that the treatment's effects are lasting for a good amount of time, which is what doctors and patients hope for.
This term refers to the middle value in the range of times it took for patients to first see a response to the treatment. In the case of AMTAGVI, the median time to initial response was 1.5 months, with the quickest response being at 1.3 months and the slowest at 4.2 months. This means that half of the patients started seeing their tumors reduce or go away at or before 1.5 months after starting the treatment. It gives an idea of how quickly you might expect to see results from the treatment.
Out of the original 160 people who received the treatment, ultimately only 73 patients were a part of the main analysis of how well AMTAGVI worked, less than half of the original.
So ORR was at 31.5% who experienced a reduction in their tumor with a confidence interval of 21.1% - 43.4%. Considering the sample size being so low, I am not sure I would be too amazed by these results. Especially when we consider the rates of adverse reaction which we will get to next. Let’s break this down a bit further first.
3 out of the 73 patients (4.1%) complete response rate had their cancer disappear.
20 out of the 73 (27.4%) partial response rate, had tumor shrink
The number (4.1, NR) tells us the shortest observed duration was 4.1 months, and 'NR' means 'not reached,' indicating that the end point of the response period hadn't been reached for many patients by the time the data were analyzed.
Patients with DoR ≥ 6 months: More than half of the patients (56.5%) or 13 patients who responded to the treatment had their cancer stay smaller for at least 6 months.
Patients with DoR ≥ 9 months: Nearly half (47.8%) or 11 patients who responded to the treatment had their cancer stay smaller for at least 9 months.
Patients with DoR ≥ 12 months: A little less than half (43.5%) or 10 of the patients who responded had their cancer stay smaller for at least 12 months.
ADVERSE REACTIONS
Let’s breakdown the adverse reactions observed in the trial. Now remember all of these with the exception of Febrile neutropenia are in addition to the conditions the FDA issued a “black box” warning over. This trial started with 160 people, 156 of which ended up being treated with AMTAGVI. Here are the results for adverse reactions observed by type.
This is accounting for adverse reactions in the total amount of individuals who received the infusion. As you’ll recall, only 73 were included in the final analysis.
These abnormalities were observed from the time of AMTAGVI infusion to 6 months afterward. Grades 3 and 4 are clinically significant and typically require intervention. (N=156) so obviously many experienced multiple of these conditions.
Thrombocytopenia - 122 people (78.2%): A low platelet count, which can lead to increased bleeding and bruising.
Neutropenia - 108 people (69.2%): A low count of neutrophils, a type of white blood cell, leading to increased risk of infection.
Anemia - 91 people (58.3%): Low red blood cell count, which can cause fatigue, weakness, and shortness of breath.
Leukopenia - 73 people (46.8%): Low white blood cell count in general, which also increases infection risk.
Lymphopenia - 66 people (42.3%): A reduced count of lymphocytes, another type of white blood cell, leading to a compromised immune system.
Hypophosphatemia - 40 people (25.6%): Low levels of phosphate in the blood, which can lead to muscle weakness, respiratory failure, and heart problems.
The two tables following the package insert information provided below, breakdown the reaction type that led to death and overall deaths.
I struggle to put myself in the shoes of the researchers and regulators who worked on this. Who after seeing all of this data on deaths and adverse events can declare these results a success. Fast tracked nonetheless by the FDA, a trend that we are seeing increase rapidly post-covid.
It is so urgent that we need to get this stuff into people’s bodies asap, significantly increasing their short term mortality risk, this according to their own data and then lovingly charging them $515K for the privilege. After all, “we warned you.”
Conflicts of Interests and the Study Authors
Buried under hyperlinks and almost deliberately difficult to navigate to are conflict of interest statements. Each author listed has to provide what they feel could be perceived as a conflict of interest. Meaning some other source of income or association that given the study subject could potentially have influenced them in some way.
Perhaps a charitable interpretation of data, a study design flaw, a questionable methodology, potential financial incentives or social incentives. The list of potential conflicts is too numerous to print here.
With that under consideration and also keeping in mind the data we just went through, I invite you to get to know 21 authors who are credited with the studies while weighing their COI’s with the data as well as the potential career/financial incentives. After you’ve done so, ask yourself, “if I had this stage of melanoma, how confident would I be accepting this treatment?”
The story is yet to be told since it has already been approved based on short term data. I guess the public is the price gauged guinea pig at this point and that my friends, is the point.
Author Conflicts of Interest Statements
JASON CHESNEY
Consulting or advisory role with Amgen.
Research funding from Replimune, Amgen, Iovance Biotherapeutics, and Fate Therapeutics.
KARL D. LEWIS
Received honoraria from Array BioPharma and Iovance Biotherapeutics.
Consulting or advisory role with Array BioPharma, Merck, Roche, Regeneron, Sanofi, Iovance Biotherapeutics, and Nektar.
Research funding from Roche/Genentech, Merck, Array BioPharma, Incyte, Nektar, Iovance Biotherapeutics, Bristol-Myers Squibb, Kartos Therapeutics, OncoSec, Regeneron, Alkermes, Neon Therapeutics, Ultimovacs, Senhwa Biosciences, Replimune, Amgen, and Seagen.
Travel, accommodations, and expenses from Merck, Roche/Genentech, Regeneron, Neon Therapeutics, and Alkermes.
Uncompensated relationships with Roche/Genentech and Regeneron.
HARRIET KLUGER
Consulting or advisory role with Bristol-Myers Squibb, Clinigen, Shionogi, Chemocentryx, Calithera, Signatera, GigaGen, GI Reviewers, and Merck.
Research funding from Apexigen, Bristol-Myers Squibb, and Merck.
OMID HAMID
Received honoraria and consulting or advisory role with numerous pharmaceutical companies, including Aduro, Akeso, and Amgen.
Research funding from several companies, including Arcus, Aduro, and Amgen.
Speaker’s bureau for Bristol-Myers Squibb, Novartis, Pfizer, and Sanofi Regeneron.
Eric Whitman
Consultant or advisory role with Merck.
Research funding received as site principal investigator for multiple studies.
Speaker’s bureau for Bristol-Myers Squibb, Regeneron, and Castle Biosciences.
Sajeve Thomas
Honoraria, consulting or advisory role, research funding, speaker’s bureau, and covered travel expenses with companies like Bristol-Myers Squibb and Merck.
Martin Wermke
Received honoraria from Novartis, Pfizer, and Roche.
Consulting or advisory role with a list of companies including Bristol-Myers Squibb and Novartis.
Research funding from Roche.
Travel and expenses from Pfizer, Bristol-Myers Squibb, AstraZeneca, and others.
Mike Cusnir
Speaker’s bureau participation with Sirtex Medical.
Evidio Domingo-Musibay
Received honoraria from Castle Biosciences.
Consulting or advisory role and speaker’s bureau with Regeneron.
Research funding from Clinigen.
Giao Q. Phan
Honoraria from IBSA.
Consulting or advisory role with Acella, Amneal, and Terns.
Patents, royalties, or other intellectual property with Virginia Commonwealth University.
John M. Kirkwood
Consulting role with numerous companies including Amgen, Bristol-Myers Squibb, and Merck.
Research trial support to institution from companies such as Amgen, Bristol-Myers Squibb, and Novartis.
Jessica C. Hassel
Received honoraria for talks from various companies including Almirall and Amgen.
Advisory boards for companies such as GSK and MSD.
Research funding from Bristol-Myers Squibb, Sun Pharma, and Sanofi.
Travel, accommodations, and expenses from Sun Pharma.
Marlena Orloff
Consulting or advisory role with TriSalus, Immunocore, Ideaya, and Delcath.
Her husband is an employee of GSK.
James Larkin
Received honoraria from companies including Eisai and Novartis.
Consulting or advisory role with companies such as iOnctura and Merck.
Research funding from Bristol-Myers Squibb, MSD, Novartis, and others.
Travel, accommodations, and expenses from Pierre Fabre, Roche, and GSK.
Jeffrey Weber
Owns stock or other ownership interests in Biond, Instil Bio, OncoV4, and Evaxion.
Honoraria and consulting or advisory role with companies including Bristol-Myers Squibb and GSK.
Research funding from Bristol-Myers Squibb, Moderna, Merck, and others.
Patents, royalties, or other intellectual property with Moffitt Cancer Center and Biodesix.
Andrew J.S.Furness
Consulting or advisory role with Immunocore and GSK.
Speaker’s bureau participation with Bristol-Myers Squibb, Ipsen, and Eisai.
Travel, accommodations, and expenses from ESMO.
Nikhil I. Khushalani
Owns stock and other interests in companies like Bellicum Pharmaceuticals.
Consulting or advisory role with several companies including Bristol-Myers Squibb and AstraZeneca.
Research funding from Bristol-Myers Squibb, Merck, Novartis, and others.
Theresa Medina
Consulting or advisory role with companies including Merck, Bristol-Myers Squibb, and Iovance Biotherapeutics.
Michael E.Egger
Consultant or advisory role with Iovance Biotherapeutics.
Research funding from SkylineDx.
Friedrich Graf Finckenstein, Madan Jagasia, Parameswaran Hari, Giri Sulur, Wen Shi, and Xiao Wu
Are employees of Iovance Biotherapeutics and hold stock and/or stock options.
FGF is in a leadership position at Iovance Biotherapeutics; owns stocks from other biotech and pharmaceutical companies; and holds patents or other intellectual property.
PH additionally received honoraria and has been paid for consultant or advisory roles by various companies and received travel accommodations from others.
Amod Sarnaik (Corresponding Author)
Received honoraria and consulting or advisory roles with Iovance Biotherapeutics and other consultancies.
Holds patents, royalties, or other intellectual property with institutions like Moffitt Cancer Center.
Notice how tedious they make it to extract COI’s in a easily readable form.
We The Free is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.





















advanced melanoma is what they expect to arise from the mRNA vaccine.....it is from the latest study in April 2024 that was mentioned, as well as prostate cancer..and head and neck cancers. this makes a lot of sense since COVID-19 seem to dwell in the parts of the body with the lowest temperature
Thank you Josh! Great work!